知识分享|EMA药品、活性物质、辅料和内包装灭菌指南翻译与解读(二)
4.1.2. Dry heat sterilization
干热灭菌
Time and temperature of the sterilisation cycle and a bioburden limit should always be stated.
应说明灭菌周期的时间和温度以及生物负荷限度。
For sterilisation using a reference condition of the Ph. Eur. 5.1.1 (a minimum of 160 °C for at least 2 h), the validation data for the sterilisation cycle is not required to be submitted in the quality dossier. For sterilisation cycles with time and/or temperature lower than the reference conditions of the Ph. Eur., physical and biological validation of the sterilisation cycle should be provided to demonstrate an SAL of ≤10-6, as described in Ph. Eur. 5.1.1. The SAL of such a sterilisation process should be calculated from the maximum bioburden per container.
对于使用 Ph. Eur. 5.1.1 的参考条件 (≥160℃至少2小时 ) 进行灭菌, 灭菌周期的验证数据不需要在质量档案中提交。对于时间和温度低于药典参考条件的灭菌周期,应提供灭菌周期的物理和生物验证, 以证明 SAL≤10-6。这种灭菌工艺的SAL应根据每个容器的最大生物负荷计算。
Where required, sufficient validation data should be submitted to demonstrate that an SAL of ≤10-6 is obtained for all containers. The data submitted should include at least, but is not limited to:
必要时,应提交足够的验证数据, 以证明所有容器都能获得≤10-6 的 SAL。提交的数据至少应包括但不限于:
Load mapping of the chamber and load mapping distribution of the items in the chamber (including the slowest to heat locations) – summary or confirmation of performance;
腔体的装载布局和腔体中物品的装载布局图(包括加热最慢的位置)- 性能确认总结;
Physical and biological cycle effect confirmation summary of at least three sterilisation runs ensuring:
至少3次灭菌周期的物理和生物性能确认总结, 以确保:
Demonstration that the sterilisation load in the steriliser chamber achieves the specified cycle parameters, including time, temperature, and, lethality;
证实灭菌器腔体中的装载达到了规定的循环参数, 包括时间、温度、致死率等;
Acceptable temperature differences between temperature sensors in the load;
负载中温度探头之间可接受的温度差异;
Acceptable lethality variability within the load;
负载内杀灭率可接受的波动;
Relationship between physical and biological validation.
物理和生物学验证之间的关系。
For the biological validation, a biological indicator as described in Ph. Eur. chapter 5.1.2 should be used.
对于生物学验证, 应使用Ph. Eur. 5.1.2章节所述的生物指示剂。
A maximum bioburden limit of 100 CFU/100 g or 100 CFU/100 ml would be acceptable for parenteral finished product formulations without further justification. For active substances and finished products that are not used for parenteral administration, a maximum total bioburden limit of 10 CFU/g or 10 CFU/ml is acceptable without further risk based justification. Other testing regimes and limits to control bioburden at the defined level should be justified. A justified bioburden limit should also be established for empty containers.
对于注射用制剂处方, 最大微生物负荷限制为 100CFU/100g 或 100 CFU/100 毫升是可以接受的,不需要进一步论述。对于非注射用制剂处方和活性物质, 最大微生物负荷限度为 10 CFU/g或 10 CFU/ml 是可以接受的,不需要进一步的风险论证。其他用以控制微生物负荷在规定水平内的方法和限度应进行论证。对于任何空的容器,也应建立合理的生物负荷限度。
Dry heat at temperatures of greater than 220 °C for a validated time is frequently used for both sterilisation and depyrogenation of glassware and other heat-resistant container materials e.g. aluminium crimps. In this case, demonstration of a 3 log reduction in heat-resistant endotoxins can be used as validation criteria.
在经过验证的时间内,温度高于220°C的干热灭菌经常被用于玻璃器皿和其他耐热容器材料,比如压制铝的灭菌和去热原。在这种情况下, 证明能够将耐热内毒素下降3个log等级作为验证的标准。
【解读】
对于注射用制剂、非注射用制剂,和原料药的微生物负荷的限度要求基本上都是行业内普遍接受的标准。虽然这个指南说不需要进行进一步的风险评估和合理化解释,但是说实话,考虑到产品的差异性以及微生物种类的差异性,这样的生物负荷的限度要求是值得推敲的。测试样品的数量规定也是一个很大的问题。这些都是实际操作中可能带来争议的地方,也会给样品的代表性带来质疑的空间。对于负责的企业而言,最好让自己的微生物专家,结合产品的实际和生产制造的实际,潜在微生物种类的实际,进行周全的风险评价,然后制订符合自己要求和实际的标准还是我们最佳的选择。
干热温度设定的底线是220°C, 这个温度点的设定基础是什么?指南没有解释,更加常见的还有250°C这个点。就内毒素而言,干热破坏的温度点250°C或许更加常见。当然时间的差异和温度的高低直接相关联,所以指南要求对时间进行验证。
4.1.3. Ionization radiation sterilization
电离辐射灭菌
For this method of sterilisation, the reference absorbed dose is ≥25 kGy. Other doses may be used to achieve an SAL ≤10−6, if justified and validated.
对于这种灭菌方法, 参考吸收剂量为≥25 kGy。如经论证和验证,也可以使用其他剂量以达到SAL≤10-6。
Data as requested in Note for Guidance “The use of Ionization Radiation in the Manufacture for Medicinal Products” and in compliance with Ph. Eur. chapter 5.1.1 should be provided. Relevant guidance in establishing the radiation dose other than 25 kGy is available in ISO standard 11137.
应提供“药品生产中电离辐射使用指南”中要求的数据, 并符合Ph. Eur 5.1.1 章节的要求。ISO 11137标准提供了确定 25 kGy 以外的辐射剂量的相关指导。
Where any requirements in ISO 11137 are in contradiction to requirements stated in any Note for Guidance issued by the EMA or Ph. Eur. monograph, the requirements of the Ph. Eur. and the Note for guidance apply.
如果 ISO 11137标准中的任何要求与 EMA 或 Ph. Eur. 专论发布的任何指导说明中的要求相抵触, 则应使用 Ph. Eur. 和医药指南的要求。
【解读】
这一规定其实表明了EMA对于ISO相关标准的态度。对于采用电离辐射灭菌的很多企业实际采用的是合同制造企业进行的操作,这些合同组织很多时候不会单纯的服务医药行业,所以更多的会在体系中遵循ISO标准,对于医药行业的QA在进行这类合同组织审计的时候,不能因为对方声称符合ISO标准就对其放松要求,要仔细核对细节,确保ISO标准和要求和现有的EMA医药指南和EP药典的相关规定相冲突。
4.1.4. Gas sterilization
气体灭菌
4.1.4.1 General considerations
通则
Generally, gas sterilisation is only acceptable if no other method of sterilisation is possible. Gas sterilisation provides sterilisation of the surface of materials. It is mainly employed for sterilising packaging materials and equipment, and has therefore only been included in the decision tree for containers. To ensure adequate sterility, sufficient penetration by gas and moisture is essential. This should be followed by a purging process to ensure that any residues of gas or related transformation by-products are below concentrations that could give rise to toxic effects during use of the finished product. The effectiveness of the purging process should be demonstrated.
一般来说, 只有在没有其他灭菌方法的情况下, 气体灭菌才是可以接受的。气体灭菌可对材料表面进行灭菌。它主要用于对包装材料和设备进行灭菌, 因此只包括在容器的决策树中。为了确保足够的无菌性, 确保气体和水分能够充分渗透是非常重要的。气体灭菌结束后应进行清除过程以确保灭菌气体或其相关的转化形成的副产品的任何残留物低于可能提高成品使用过程中的毒性影响的浓度。应证明清除过程的有效性。
Gas sterilisation of porous compounds, such as dry powders, is not acceptable unless other methods of sterilisation are not feasible and its use is scientifically justified. Prior to the gas sterilisation, the active substance or excipient should be sterile filtered and crystallised under aseptic conditions to minimise bioburden and entrapment of micro-organisms within the crystals. Convincing evidence should be provided demonstrating that the material to be sterilised is not susceptible to compression preventing gas and moisture penetration during sterilisation.
对多孔化合物 (如干粉) 进行气体灭菌是不可接受的, 除非其他灭菌方法不可行且使用该方法经科学论证。在气体灭菌之前, 活性物质或辅料应在无菌条件下进行除菌过滤和结晶, 以最大限度地减少微生物在晶体中的吸收和包裹。应提供令人信服的证据, 证明待灭菌的材料在灭菌过程中不易挤压,防止气体和水分渗透。
A description of the apparatus, quantitative data on gas(es) to be used, the bioburden prior to sterilisation, the time of exposure to the gas, the temperature and humidity prior to and during each step of the sterilisation cycle, and, if applicable, the conditions for the removal of any toxic gas residues should be provided. Humidity used for the preconditioning and/or conditioning of the material to be sterilised shall be generated by clean steam. These conditions should be monitored by appropriate in-process controls with justified acceptance criteria. The process should be developed and validated in compliance with Ph. Eur. 5.1.1 and 5.1.2. A risk assessment with regards to residual toxic impurities should be conducted and a control strategy should be provided where applicable. The requirements should be in accordance with the requirements of ICH M7 “Assessment and control of DNA reactive (mutagenic) impurities in pharmaceuticals to limit potential carcinogenic risk”. Even if the relevant product is outside the scope of that guideline, its limits for highly toxic impurities could be applied.
应提供设备描述、要使用的气体的定量数据、灭菌前的生物负荷、接触气体的时间、灭菌周期每一步开始前和期间的温度和湿度, 以及,适当时,清除任何有毒气体残留的条件。用于对待灭菌材料进行预处理和/或调节的湿度应通过清洁蒸汽产生。这些条件应通过适当的过程控制和合理的接受标准进行监测。应按照 Ph.Eur. 5.1.1 和5.1.2 章节制定和验证这一程序。应对残留有毒杂质进行风险评估, 并酌情提供控制战略。这些要求应符合 ICH M7指南 “评估和控制药品中的 DNA 反应性 (诱变) 杂质以限制潜在致癌风险”。即使相关产品可能不适用于该指南的范围, 该指南中对剧毒杂质的限度也是可以使用的。
Results of the process validation should demonstrate an SAL of ≤10-6.
工艺验证的结果应证明SAL ≤10-6。
The effectiveness of the process should be routinely checked for every batch confirming that the process parameters and biological indicators are all within their acceptance criteria and by sterility testing. Parametric release is not acceptable for gas sterilisation (according to Ph. Eur. chapter 5.1.1).
日常应通过确认工艺参数和生物指示剂都在其接受标准范围内和无菌测试来检查每一批工艺的有效性。参数放行对于气体灭菌是不可接受的 (参考Ph. Eur. 5.1.1 章节)。
【解读】
采用气体对产品进行灭菌永远是最后的选择,不得以采用的选择,因为气体和灭菌目标物往往出于不同的状态,而且比蒸汽灭菌更加复杂的是因为温度的限定往往杀菌作用是和接触时间和接触浓度相关的,而接触浓度会带来潜在的灭菌气体和目标物之间的化学反应,这些要素都让灭菌过程更加复杂,也给产品引入新的杂质机会。杀菌气体,由于其固有的杀菌特性决定了其对于正常人体细胞的毒性,所以其残留是不被允许的,给消毒后的残留检测标准和检测方法有带入了新的问题。这些都是气体灭菌不被首先考虑的科学基础。
在实际操作中,当气体灭菌的决策被最后采纳后,我们强烈的建议公司要耐心的进行各项基础的实验去收集尽可能多的信息和数据,然后根据这些数据和信息来制订科学完善的灭菌工艺流程和相关的参数,并确保操作人员严格按照相关的流程和操作细节进行生产操作。如果你是审计人员,工厂存在这个类型的操作的时候,不得不高度重视我们在这里提到的这些环节了。
4.1.4.2 Ethylene oxide sterilization
环氧乙烷灭菌
Ethylene oxide (ETO) sterilisation processes should be developed and validated in compliance with Ph. Eur. 5.1.1 and 5.1.2. Relevant guidance in establishing the sterilisation process cycle parameters and validation is available in ISO standard 11135.
应按照 Ph. Eur 5.1.1 和5.1.2 章节开发和验证环氧乙烷 (ETO) 灭菌工艺。ISO 11135标准提供了在确定灭菌工艺循环参数和验证方面的相关指导。
ETO is a gas which is highly toxic. ETO sterilisation is generally only acceptable if no other method of sterilisation is possible. The risk assessment should consider the residual known genotoxic impurities (such as ETO and halogenated ethylenehydrines). This should be evaluated in accordance with the requirements of ICH M7 “Assessment and control of DNA reactive (mutagenic) impurities in pharmaceuticals to limit potential carcinogenic risk”, unless the relevant product is outside the scope of that guideline. For products outside the scope of ICH M7, the applicant should apply limits for highly toxic impurities in accordance with ICH M7, or the acceptance criteria stated in Table 2, whichever is most appropriate.
ETO 是一种高毒性气体。ETO 灭菌通常只有在没有其他灭菌方法的情况下才可接受。风险评估应考虑已知的残留遗传毒性杂质 (如 ETO 和卤化乙二氢)。应根据 ICH M7 指南"评估和控制药品中的 DNA 反应性 (诱变) 杂质以限制潜在致癌风险" 的要求对此进行评估, 除非相关产品不在该指南的范围之内。对于 ICH M7 范围以外的产品, 申请人应根据 ICH M7指南 或表格2中规定的接受标准对剧毒杂质实施限制, 以最适当者为准。
For empty containers intended to be filled with aqueous products, (e.g. prefilled syringes), the need to justify the use of ETO in the sterilisation of the container prior to filling can be waived, as the degradation kinetics of ETO in an aqueous medium have been sufficiently demonstrated. However, the levels of toxic residues (ETO and halogenated ethylenehydrines) in the finished product need to fulfil the requirements of ICH M7, or the limits stated in Table 2 below, as applicable.
对于打算装满水溶性性产品的空容器 (例如预填充注射器), 可以免除对灌装前使用ETO对容器灭菌的论证,因为ETO在水介质中的降解动力学已经充分证明。但是, 成品中毒性残留物 (ETO 和卤化乙基氢) 的含量需要满足 ICH M7 的要求,或者适当时,满足下文表格2所述的限度。
Table 2 Limits for toxic gas residues from Ethylene Oxide sterilisation where the ICH M7 limits do not apply
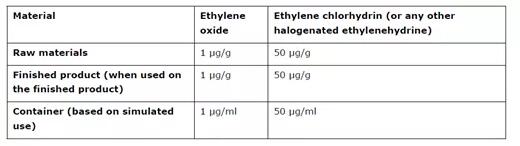
表格2环氧乙烷灭菌产生的有毒气体残留限值(不适用ICH M7指南的情况下)
4.1.5. Sterile filtration
除菌过滤
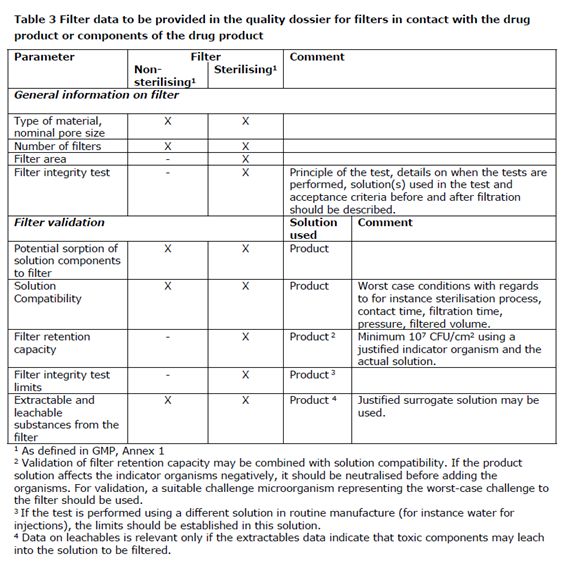
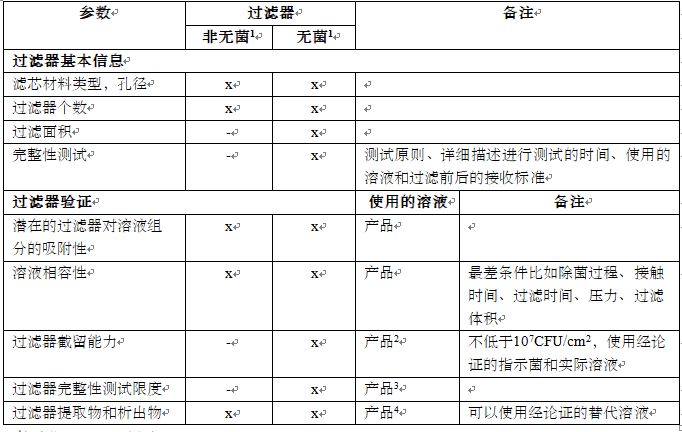
The filter data to be provided in the quality dossier is summarized in Table 3.
在质量文件中应提供的过滤器的数据总结在表格3中。
The integrity of the sterilised filter should be verified by testing before use unless specifically justified and validated, and should be verified by on line testing immediately after use. Nominal pore sizes of 0.22 µm or less are acceptable without further justification, in accordance with Ph. Eur.
除非被特殊论证和验证过,除菌过滤器的完整性应在使用前通过测试进行确认, 并应在使用后立即通过在线测试进行确认。根据 Ph. Eur., 在没有进一步理由的情况下, 正常的0.22μm 或以下的孔径是可以接受的。
For routine commercial manufacturing, bioburden testing should be performed on the bulk solution immediately before sterile filtration.
对于日常的商业生产, 应在除菌过滤前立即对溶液进行生物负荷测试。
In most situations, a limit of NMT 10 CFU/100 ml (TAMC) would be acceptable for bioburden testing. If a pre-filter is added as a precaution only and not because the unfiltered bulk solution has a higher bioburden, this limit is applicable also before the pre-filter and is strongly recommended from a GMP point of view. A bioburden limit of higher than 10 CFU/100 ml before pre-filtration may be acceptable if this is due to starting material known to have inherent microbial contamination. In such cases, it should be demonstrated that the first filter is capable of achieving a bioburden of NMT 10 CFU/100 ml prior to the last filtration. Bioburden should be tested in a bulk sample of 100 ml in order to ensure the sensitivity of the method. Other testing regimes to control bioburden at the defined level should be justified.
在大多数情况下, 生物负荷测试可以接受≤10 CFU/100 ml (总需氧菌计数) 的限度。如果添加预过滤器仅为预防措施, 而不是因为待过滤溶液生物负荷过高,则此限度也适用于预过滤器之前,并且从GMP角度考虑也是强烈建议如此。如果由于起始原料已知具有固有微生物污染, 在预过滤前生物负荷限度高于10 CFU/100 ml 也是可以接受的。在这种情况下, 应证明经过第一步过滤器后在最后一个过滤器之前能够达到≤10 CFU/100 ml 的生物负荷。为了确保方法的灵敏性,生物负荷应该对100毫升的溶液进行检测。其他用以控制生物负荷在规定水平的测试方法应进行论证。
【解读】
这里的规定解决了长期以来的关于一级过滤器和二级过滤器接受限度的讨论和争议。从目标属性出发来解释是最好的理解的,因为生物负荷过高来通过一级二级过滤器降低本身就是悖论,因为过滤器并没有杀灭微生物本身,大量生物负荷的存在只会给过滤器本身带来更大的负担,以及使用期内的微生物风险。至于生物负荷本身的限度标准的科学性,本指南也没有进行解释,更多的情况下,最安全的做法就是根据批量、过滤器型号规格、产品特性、过滤速度、压力、甚至过滤温度来综合的评价和计算最为安全的限度。当然关于取样量,本指南强调了100毫升的底线。但是,一样这个100毫升也没有任何的科学基础。样品量和样品代表性的问题需要公司自己根据实际情况进行系统评价,批量、过程时间、溶液的物理化学状态、统计学样本量都是需要考虑的。
The maximum time between the start of bulk solution preparation and sterile filtration should be stated, minimised and appropriately supported by data. Filtration times longer than 24 hours should be justified.
应说明和最大限度减少从溶液配制到除菌过滤之间的最长时间间隔,并有数据支持。过滤时间超过24小时应进行论证。
If a sterile filtered bulk solution is not filled into the final product containers within 24 hours, the sterile filtration should, unless justified, be repeated immediately before filling. An additional bioburden test should be performed before any further bioburden reduction step after the holding time. The holding time should be adequately justified.
如果除菌过滤后的溶液未在24小时内分装入最终产品容器, 除非经论证, 否则应立即重新进行除菌过滤才能灌装。一旦超出保存时间,在任何进一步减少生物负荷的步骤之前,应进行额外的生物负荷检验。保存时间应进行充分论证。
【解读】
24小时的科学基础显然是微生物是活体的,保存时间过长,不可避免发生生物负荷的增加,除非有科学的数据证明其变化的趋势。当然,大家也都知道实际微生物的生长条件是受到温度、细菌种类、溶液组分、储罐条件等环境因素影响的。所以一样,24小时本身没有太科学的依据,所以不希望实际操作中大家把24小时这个限度做为金标准,金标准还是数据。

全部 0条评论